Phthalonitriles (PN)
Properties
Phthalonitriles (PN) are a relative new class of commercial high
performance engineering thermosets. They exhibit very high thermal
and oxidative stability, and outstanding high-temperature
properties. Infact, PNs have one of the highest decomposition
temperature of all organic thermoset polymers which can approach 500
°C (930 °F) in air.1-4
They also exhibit no Tg before decomposition when post-cured, and
thus, have no viscoelastic transition before thermal decomposition. Other important
benefits include very low water uptake, outstanding structural
integrity when exposed to excessive heat or fire, and excellent corrosion resistance. In addition, the high aromatic content and high crosslink density of the
PN thermosets yields a high char content of about 80 to 90% when
pyrolyzed to 1000° C under inert conditions. Both, the high thermal
stability and the high char yield contribute to the outstanding
high-temperature performance. However, PNs
have also some major limitations and shortcomings. For example, they are
very expensive and difficult to process, relative to conventional thermoset resins.
Recently, a new class of PN resins has been developed, the so-called
second-generation PEEK-like phthalonitriles.4 These new
resins allow for more cost-effective manufacturing due to a simpler two-step and one-pot cure and cheaper starting materials. The oligomeric resins are liquid above 70°C and polymerize to thermosets above 150°C, allowing for an ample processing temperature window.
For example, composites can be produced by well-established industrial methods such as resin transfer molding (RTM) and resin infusion molding (RIM), and by other low-cost manufacturing techniques. Furthermore, the low melt viscosity and larger processing window makes the
fabrication of thick composite parts possible where the melt has to impregnate thick fiber preforms.
SYNTHESIS
Phthalonitriles resins can be prepared by nitro-displacement of nitro-phthalonitriles with phenoxyphenols in the presence of a base. The reaction is typically carried out in a solvent such as DMF to control the temperature of the reaction.
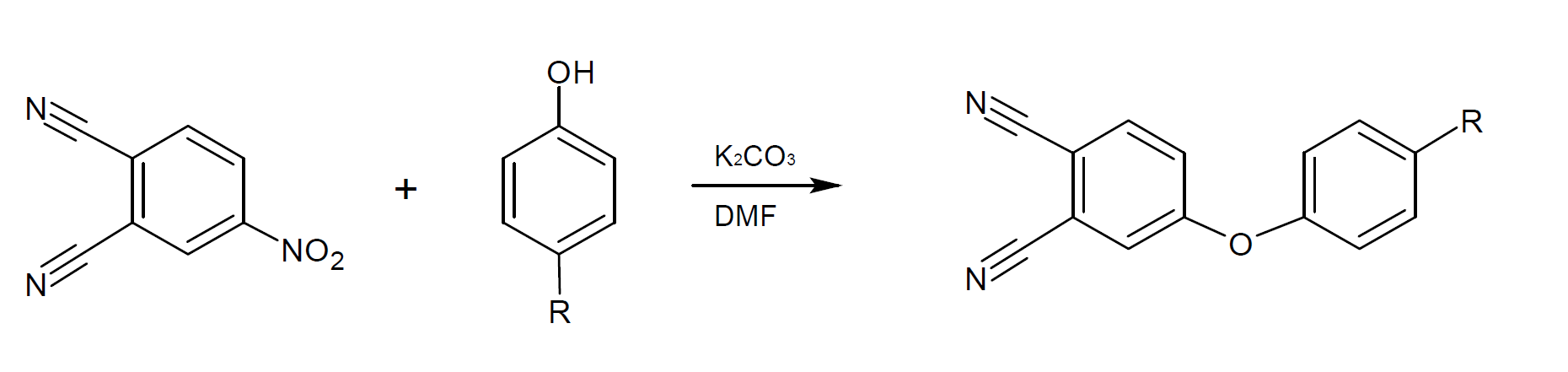
Oligomeric ether-aromatic ketone-containing phthalonitriles have been synthesized from a bisphenol and chlorobenzophenone with bisphenol always in excess to ensure termination of the oligomeric composition as a diphenolate. The phenolate terminated resin is then end-capped by the reaction above to produce the oligomeric phthalonitrile resin.2,4
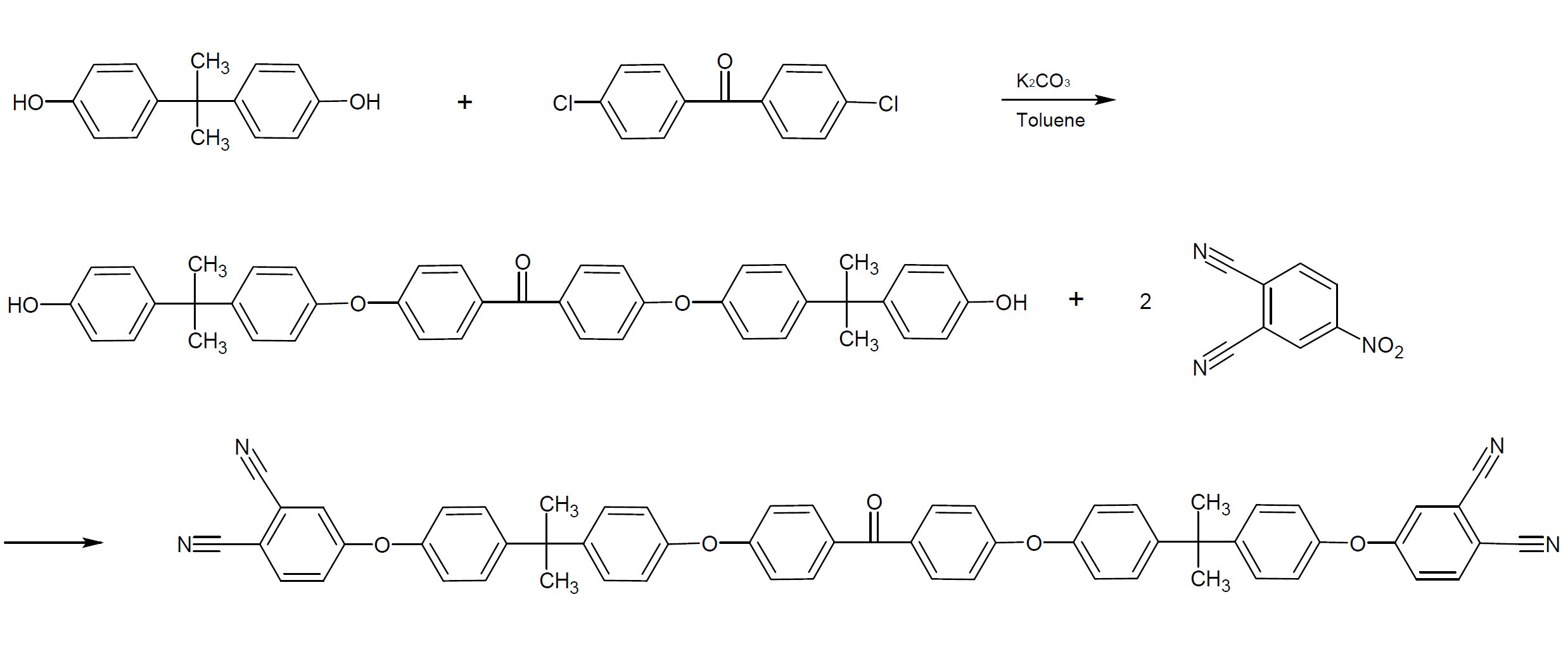
The reaction is typically carried out in a protic solvent such as toluene to control the refluxing azeotropic removal of water and the temperature of the reaction.2
A large variety of phthalonitrile monomers and oligomers containing aromatic ether, thioether, imide, ketone and sulfone linkages between the terminal phthalonitrile groups have been synthesized. These resins can be cured with a number of organic curatives including amines, strong organic acids and organic acid-amine salts, as well as metallic salts at temperatures between 200 and 300° C. A very common curative is 3-bis(3-aminophenoxy)-benzene (m-APB). The reaction with phthalonitriles leads to the formation of triazine and phthalocyanine structures.
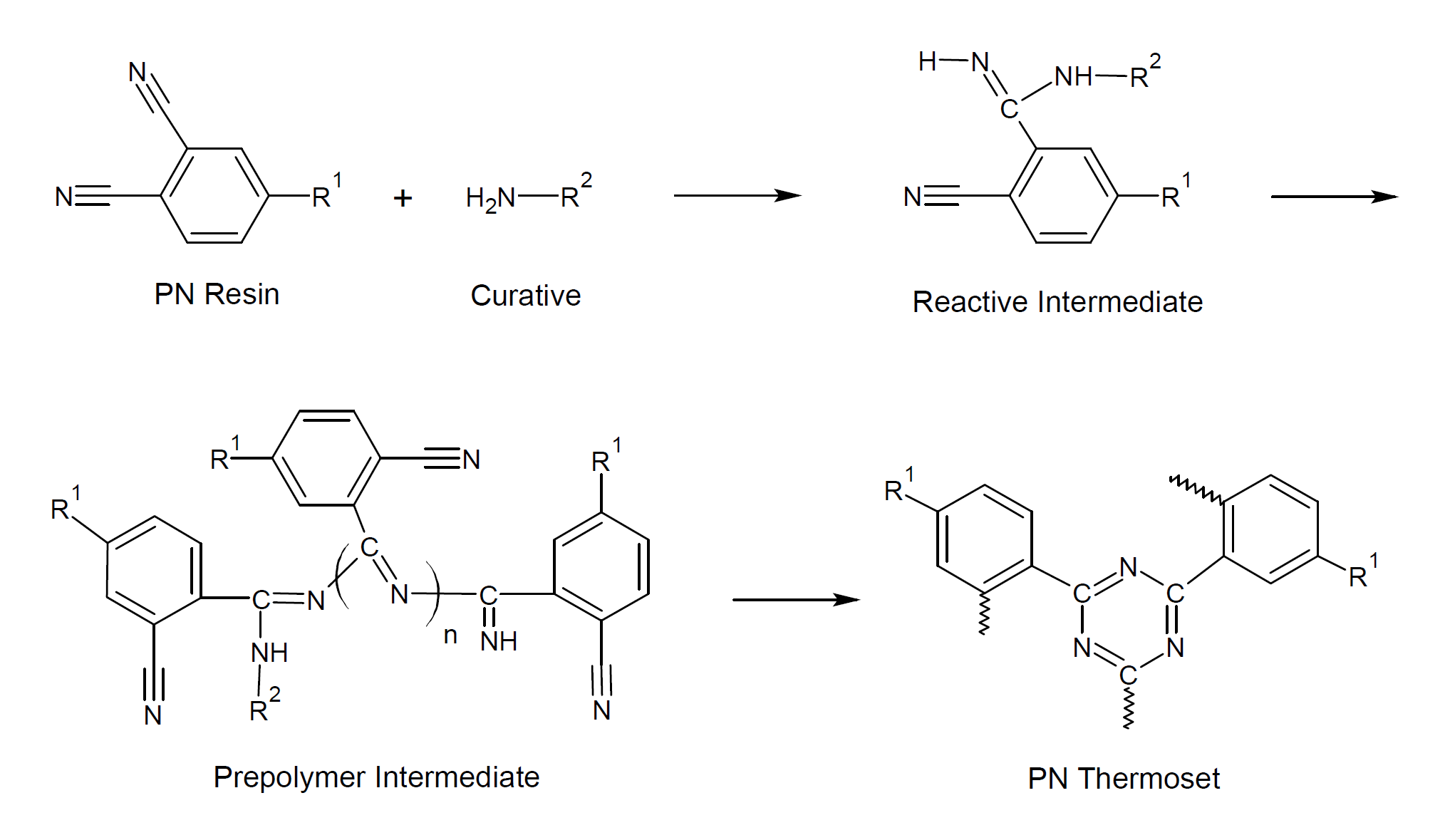
The network structure of triazine/phthalocyanine and aromatic rings linked by ketone and other groups imparts outstanding flame retardancy and chemical resistance, but also makes the polymer very rigid. To reduce the brittleness, plasticizers are often added. The plasticizer can be a reactive or non-reactive including reactive monophthalonitrile plasticizers, which reduce the crosslinking density and toughen the polymer. They also affect the thermophysical and mechanical properties such as mechanical strength and glass transition temperature.
| Diphthalonitrile | Structure of Repeat Unit |
| Bisphenol A diphthalonitrile |
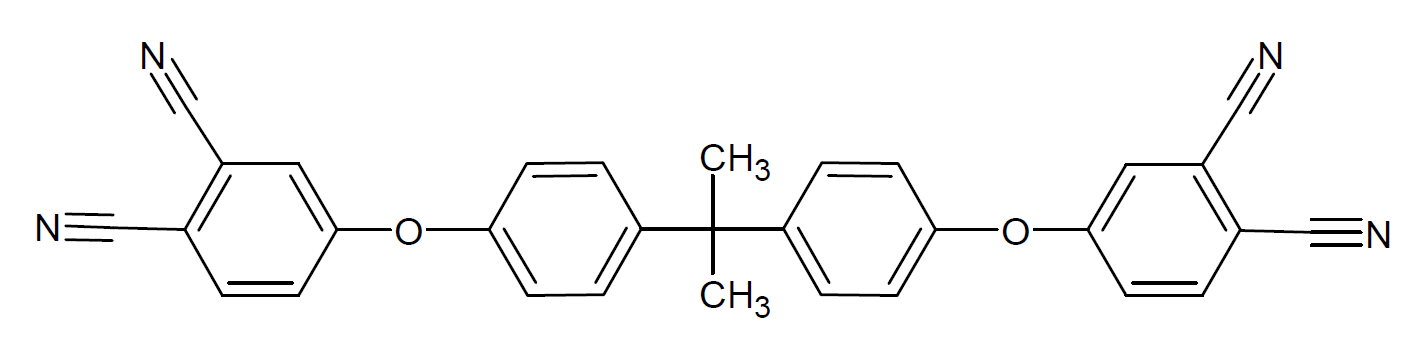
|
| Resorcinol Diphthalonitrile |
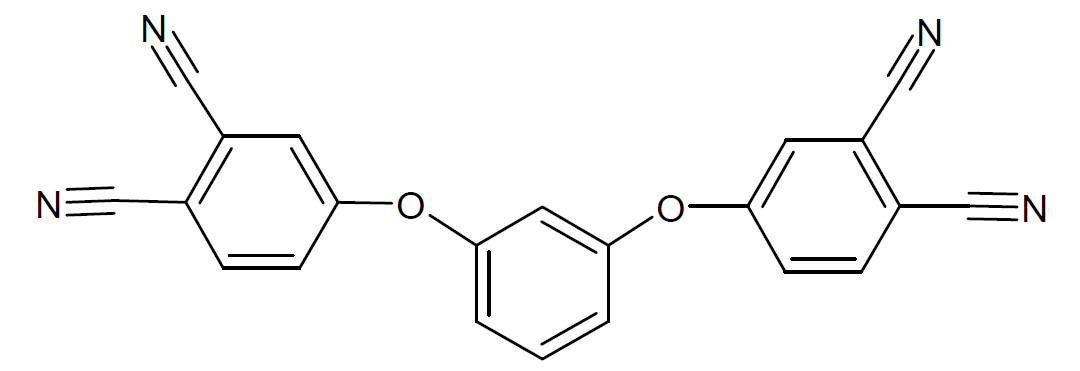
|
| Poly{[bis(4-bromophenyl) sulfone]-alt-[bisphenol-A]} diPN |
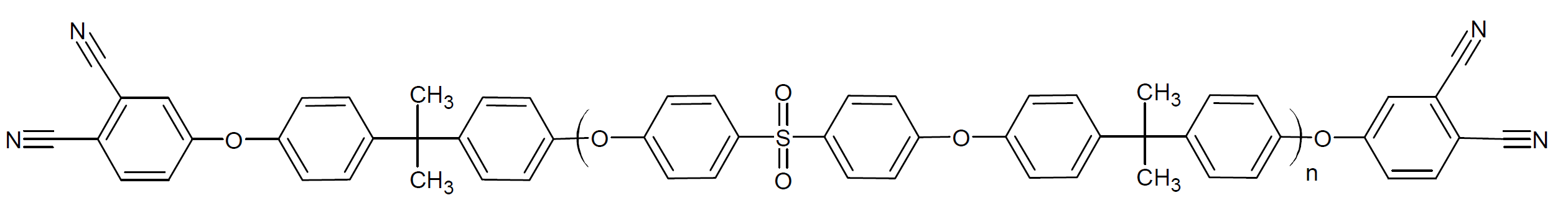
|
| Poly{[dibromobenzophenone]-alt-[bisphenol-A]} diPN |
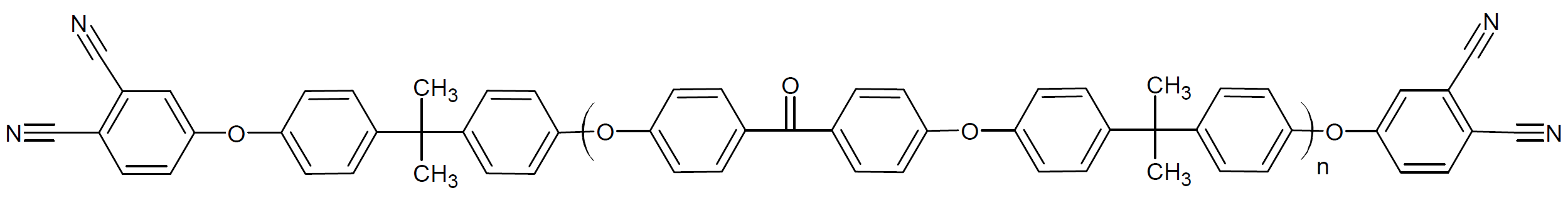
|
COMMERCIAL Phthalonitriles
High performance thermosetting phthalonitrile resins were developed and patented by the Naval Research Laboratory Chemistry Devision (NRL) who recently patented PEEK-like phthalonitrile (PN) resins.1,2 These new thermosets do not soften at temperatures as high as 932°F/500°C when initially cured at 482°F/250°C and post-cured at temperatures up to 896°F/480°C in an inert atmosphere.1 The NRL licenses these new polymer systems for commercial applications. A major manufacturer of phthalonitrile products is ITECMA. The high-temperature performance of carbon and glass fiber composites made with these resins exceeds those of most other commercial thermoset-based composites used for aerospace and other demanding applications. In fact, phthalonitriles are currently the only commercial polymeric materials that meet the MIL-STD 2031 standard for usage inside of a submarine.4
Applications
Phthalonitriles are often an excellent choice for very demanding applications where high mechanical strength in combination with high temperature and flame resistance are required or advantageous. They can replace polyimides for demanding aircraft applications and vinyl esters and epoxy resins in structural composites in the transportation industry due to their good processability and superior physical properties. Other (potential) products include high temperature adhesives and coatings, electronic packaging resins, fire-resistant garments and building materials, as well as valves, seals, bushings, bearings, wind blade components and other structural parts that have to survive in harsh and hot environments. PNs can replace metals, ceramics and other high performance materials in many applications.
PNs have one of the highest service temperatures in air. For example, some grades can withstand temperatures as high as 300 - 375°C and do not undergo thermal and oxidative decomposition at temperatures as high as 450° - 500°C.4 Because of their high price, phthalonitriles are only used when outstanding properties are required.
THERMO-PHYSICAL PROPERTIES
References and Notes
1US Patent No. 8,921,510 B1, Phthalonitrile Prepolymer Intermediates, Dec. 30, 2014
2US Patent Nos. 8,735,532; 8,859,712; 8,981,036; and 2017/0002146 A1
3 CW (Composites World), Resins for the Hot Zone, V. McConnell. 8/18/2009
4 US 2017/0002146 A1, Controlling Crosslinking Density and Processing Parameter of Phthalonitriles, Jan. 5, 2017